A quick, well tolerated, non-invasive and effective treatment for depression
BrainsWay uses Deep TMS technology, which has been tested in over 60 clinical studies for various indications in leading institutions world wide. The FDA has cleared this technology for clinical use in Major Depressive Disorder and it is currently available in the US, Europe and South America. Over 15,000 patients have already been treated with BrainsWay technology.
If you have been diagnosed with major depressive disorder (MDD) and one or more antidepressant medications haven’t worked for you, BrainsWay can help you achieve wellbeing. Each therapy session lasts approximately 20 minutes, has minimal side effects, and is covered by Medicare and most private payers.
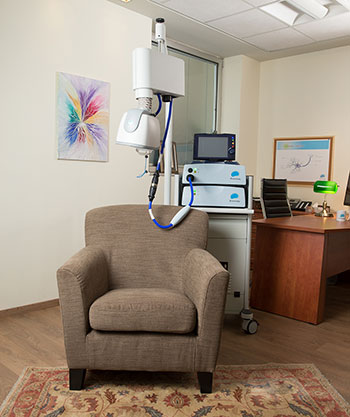
During each session, you are comfortably seated on a chair and a cushioned helmet is placed over your head. The helmet uses magnetic fields to stimulate the targeted brain area and improve depressive symptoms. The procedure requires daily sessions of 20 minutes for 4-6 weeks. As the treatment does not require hospitalization or anesthesia and entails no memory loss and no systemic side effects, you can return to your normal routine after each session.
In real-life clinical settings BrainsWay treatment was proven to be effective for 3 out of 4 patients, with a 51% remission rate and a 75% response rate no matter how many other medications were tried in the current depressive episode.
Safety Information: Patients should consult with their doctor before undergoing BrainsWay. The most common side effects include headaches and application site pain or discomfort. There is also a very rare risk of seizure associated with the treatment. Patients with metal in or around the head such as in metal plates, implants and stents, should not undergo Deep TMS treatment.